| |
Software Requirements
Microsoft Internet Explorer 5.01 or higher and Netscape Navigator 4.75 or higher are recommended. |
|
 |
J.Health Sci., 52(2), 192-197, 2006
Evaluation of the Observed Cisplatin Nephrotoxicity in Adult Cancer Inpatients: A Historical Cohort Study by Using Clinical Data Warehouse
Qiyan Zhang,a Yasushi Matsumura,*, a Tadamasa Teratani,a Sachiko Yoshimoto,a Takahiro Mineno,a Katsuhiko Nakagawa,a Munetoshi Nagahama,a Shigeki Kuwata,b and Hiroshi Takedaa
aDepartment of Integrated Medicine, Medical Informatics, Graduated School of Medicine, Osaka University, 2-15 YamadaOka, Suita City, Osaka 565-0871, Japan and bMedical Informatics Division, Totori University Hospital, 36-1 Nishi-cho Yonago City, Tottori 683-8504, Japan
Objectives: To evaluate the observed cisplatin (CDDP) nephrotoxicity in adult cancer inpatients by using Clinical Data Warehouse (CDW). Methods: A historical cohort study was conducted in Osaka University Hospital. Adult cancer inpatients (2000/1/1-2004/12/31) whose sCr level on admission < 1.2 mg/dl were divided into three groups based on the exposure of CDDP, non-exposure of CDDP but with (Non-CDDP group) or without (Control group) other anticancer agents. Patients whose sCr 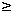 1.2 mg/dl at least once during hospitalization were considered as those having the occurrence of the nephrotoxicity and their sCr < 1.2 mg/dl on discharge as having recovered from the nephrotoxicity. After matching patients' characteristics through stratification and randomization, the rates of nephrotoxicity and the rates of recovery among the three groups were compared. Logistic regression was performed to investigate the CDDP doses - nephrotoxicity associations. Results: The rate of observed nephrotoxicity was 28.0% (127/454) in the CDDP group, 19.6% (89/454) in the Non-CDDP group and 19.4% (88/454) in the Control group, respectively (p = 0.002). The risk ratio (RR) was 1.43 (95%CI: 1.13-1.81), 1.44 (95%CI: 1.14-1.83) and 1.01 (95%CI: 0.78-1.41) between CDDP vs. Non-CDDP, CDDP vs. the control and Non-CDDP vs. the Control group. The recovery rate was 69.3% (88/127), 61.8% (55/89) and 69.3% (61/88) in the three groups, respectively (p = 0.903). The observed CDDP nephrotoxicity was dose associated (p = 0.037) but not total accumulated doses associated (p = 0.144). Conclusions: CDDP is a risk factor to the observed nephrotoxicity in our adult cancer inpatients. The observed nephrotoxicity was CDDP dose related and is considered to be reversible. 1.2 mg/dl at least once during hospitalization were considered as those having the occurrence of the nephrotoxicity and their sCr < 1.2 mg/dl on discharge as having recovered from the nephrotoxicity. After matching patients' characteristics through stratification and randomization, the rates of nephrotoxicity and the rates of recovery among the three groups were compared. Logistic regression was performed to investigate the CDDP doses - nephrotoxicity associations. Results: The rate of observed nephrotoxicity was 28.0% (127/454) in the CDDP group, 19.6% (89/454) in the Non-CDDP group and 19.4% (88/454) in the Control group, respectively (p = 0.002). The risk ratio (RR) was 1.43 (95%CI: 1.13-1.81), 1.44 (95%CI: 1.14-1.83) and 1.01 (95%CI: 0.78-1.41) between CDDP vs. Non-CDDP, CDDP vs. the control and Non-CDDP vs. the Control group. The recovery rate was 69.3% (88/127), 61.8% (55/89) and 69.3% (61/88) in the three groups, respectively (p = 0.903). The observed CDDP nephrotoxicity was dose associated (p = 0.037) but not total accumulated doses associated (p = 0.144). Conclusions: CDDP is a risk factor to the observed nephrotoxicity in our adult cancer inpatients. The observed nephrotoxicity was CDDP dose related and is considered to be reversible.
|
|

