| |
Software Requirements
Microsoft Internet Explorer 5.01 or higher and Netscape Navigator 4.75 or higher are recommended. |
|
 |
J.Health Sci., 48(5), 393-398, 2002
Investigation into the Haematologic and Hepatotoxic Effects of Rinbacin in Rats
Chudi Dioka,a Orish Ebere Orisakwe,b Onyenmechi Johnson Afonne,*, b Patrick Ugochukwu Agbasi,b David Dezi Akumka,c Chike James Okonkwo,d and Ndidi Ilondub
Departments of aChemical Pathology, bPharmacology, and dHuman Biochemistry, College of Health Sciences, Nnamdi Azikiwe University, Nnewi Campus, P.M.B. 5001, Nnewi, Anambra State, Nigeria and cNational Institute for Pharmaceutical Research and Development, Idu, P.M.B. 21, Garki, Abuja, Nigeria
An evaluation of the effects of rinbacin on the liver and blood of albino rats was carried out. Low (26.25 g/l) and high (52.50 g/l) dose levels of rinbacin were administered in the drinking water of albino rats for 13 weeks. Food and fluid intake were measured daily, and animal body weight taken weekly. Biochemical analysis of the liver function was carried out as well as some haematological parameters and histology of the liver. Results showed a significant (p 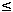 0.05) increase in all the liver function parameters tested at both dose levels. There was also an increase in packed cell volume (PCV) in the high dose group. Histological examination indicates that rinbacin at both dose sizes induced severe pathologic changes in the forms of degeneration of hepatocytes, necrosis, oedema, cellular infiltration, nuclear fragmentation and chromatinolysis. Administration of rinbacin, though could raise the PCV, may lead to hepatic damage, which might result in increased bilirubin and liver enzymes in rats. Rinbacin is toxic to the rat liver. 0.05) increase in all the liver function parameters tested at both dose levels. There was also an increase in packed cell volume (PCV) in the high dose group. Histological examination indicates that rinbacin at both dose sizes induced severe pathologic changes in the forms of degeneration of hepatocytes, necrosis, oedema, cellular infiltration, nuclear fragmentation and chromatinolysis. Administration of rinbacin, though could raise the PCV, may lead to hepatic damage, which might result in increased bilirubin and liver enzymes in rats. Rinbacin is toxic to the rat liver.
|
|

